Science: Section 3
Combustion of Fossil Fuels
Combustion of Fossil Fuels
When fuels burn they react with oxygen in the air. Air is a mixture of gases made up of mainly nitrogen and oxygen gas.
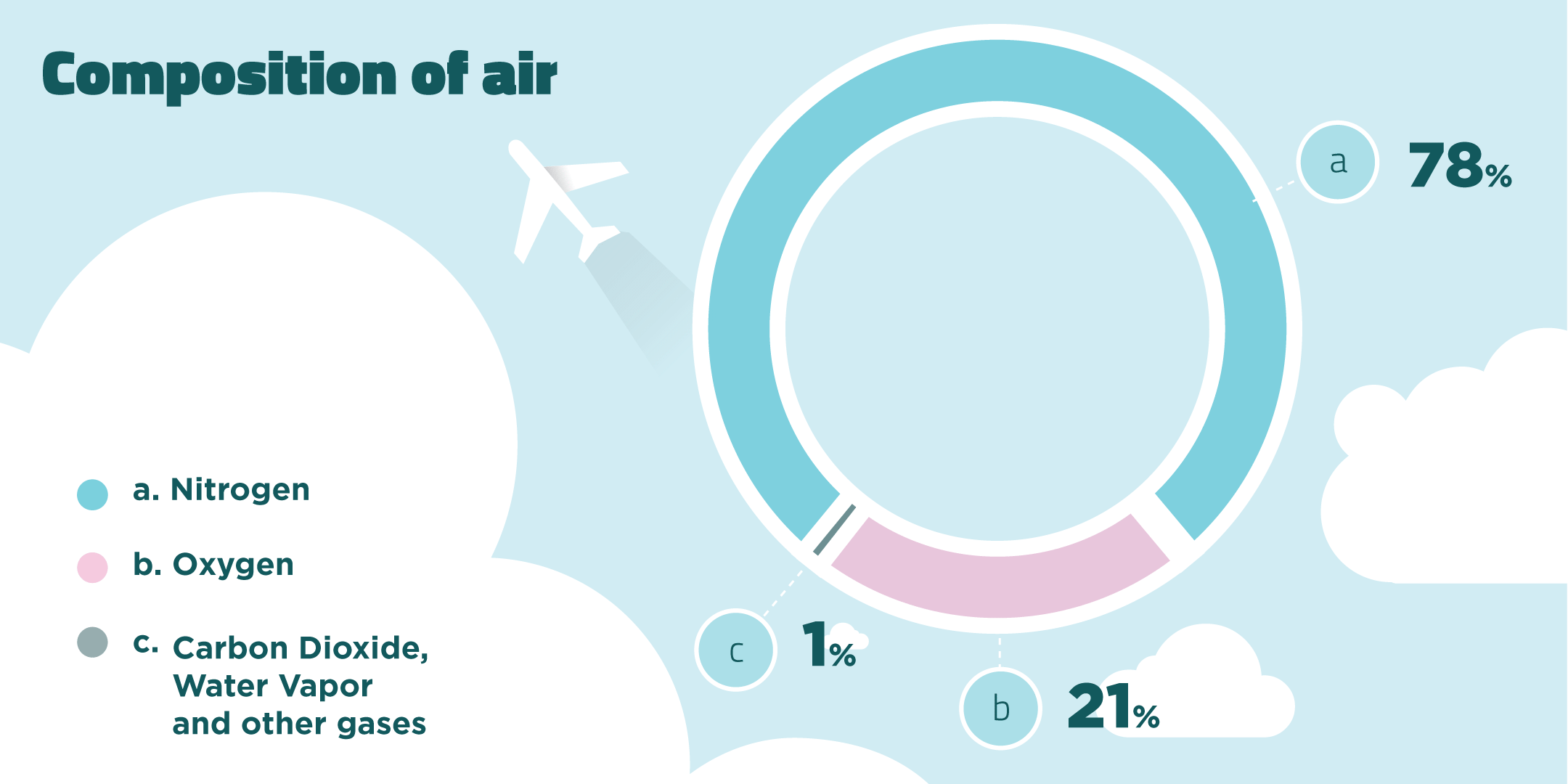
Products of Combustion
When a hydrocarbon burns in a plentiful supply of oxygen, carbon dioxide and water are produced.

This is known as complete combustion.
This can be shown by the following experiment...
Products of Combustion Experiment
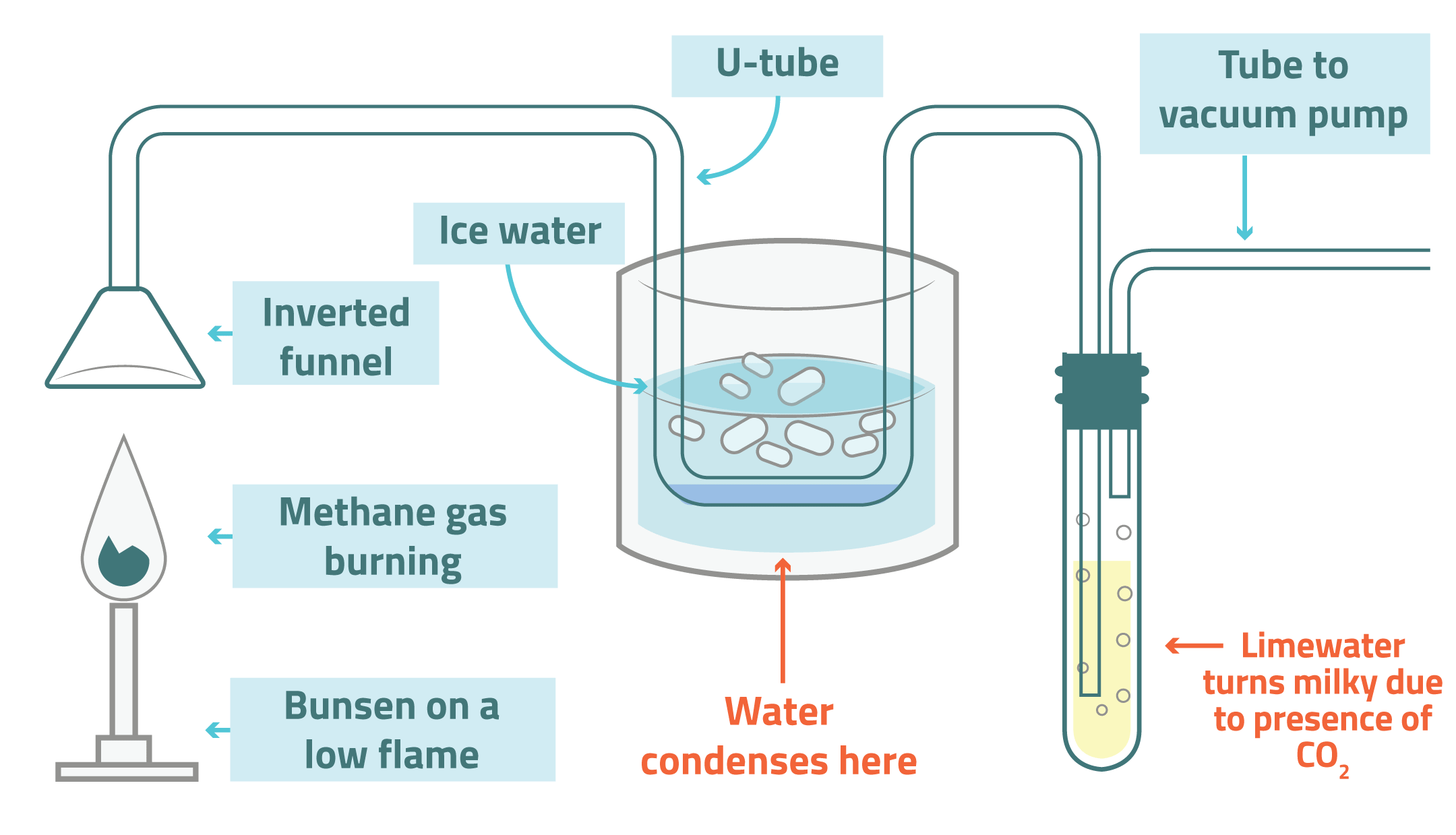
Products of Combustion Experiment
Other Products of Combustion
When a hydrocarbon burns in a limited supply of oxygen, carbon monoxide and fine particles can be produced which can affect health.

This is known as incomplete combustion.
Other Products of Combustion
- Carbon monoxide
- Fine Particles
- Nitrogen dioxide
- Sulfur dioxide
- Ozone (O3)
Science: Section 3
Combustion of Fossil Fuels
Continue to Section 4.
Close & return to main site.