Science: Section 2
Crude Oil
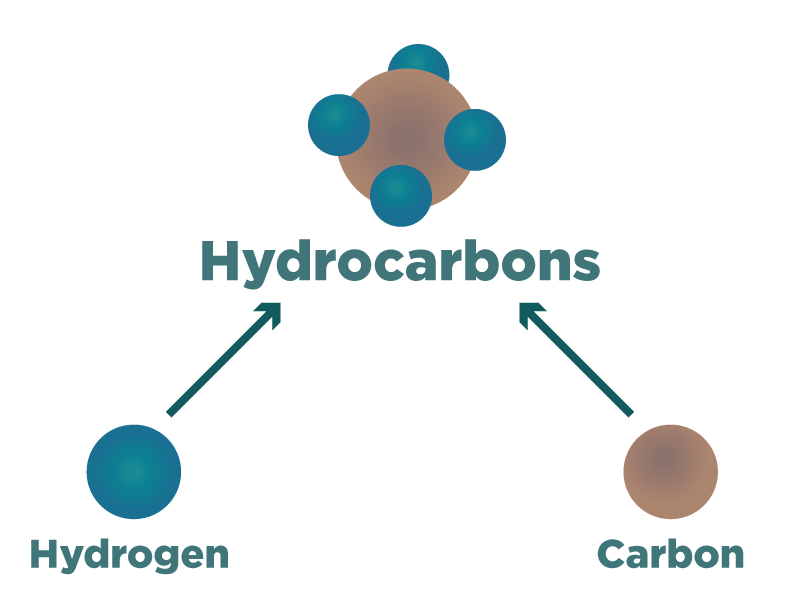
Crude oil is a black sticky substance. Hidden in it are many different substances that have essential everyday uses. Petrol, diesel, tar and many other substances that play very important roles in our everyday lives are found in crude oil.
These substances are known as hydrocarbons:-
Fractional Distillation
Fractional distillation is used to separate crude oil into fractions.
A fraction is a group of hydrocarbons all with boiling points within a specific range.
Because each fraction has its own boiling point, when the crude oil is heated each fraction will boil at different temperatures and leave the crude oil mixture as a gas. This allows them to be separated and collected.
This is done in a fractionating column.
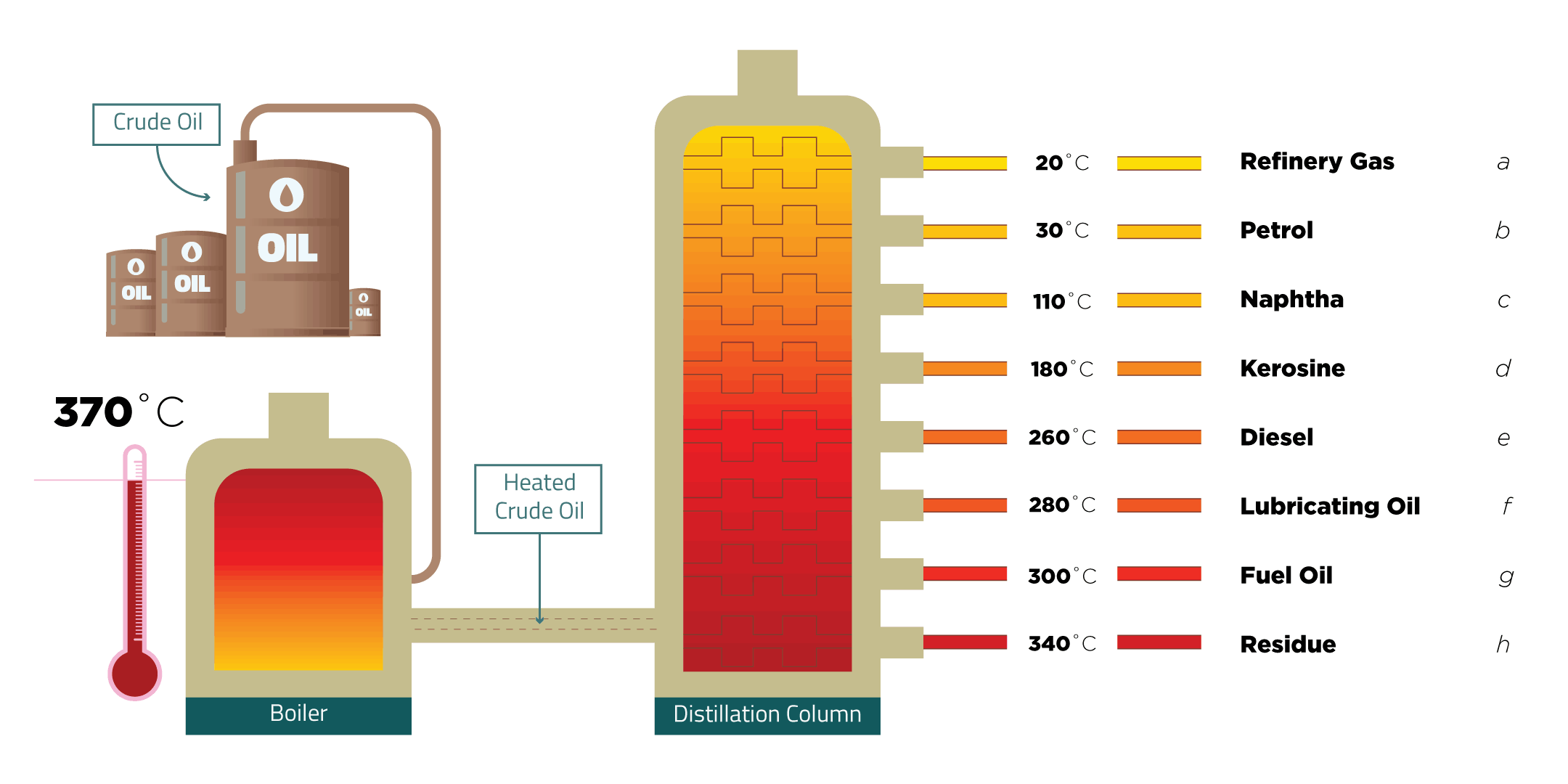

As you go up the fractionating column, the hydrocarbon molecules get smaller and have:
- lower boiling points
- lower viscosity (they flow more easily)
- higher flammability (they ignite more easily)
This means that in general hydrocarbons with small molecules make better fuels than hydrocarbons with large molecules and not just because they burn better but because they produce less fine soot particles as a result of having fewer carbon atoms in their chain.
A mnemonic can be used to help you to remember the fraction in order. Use this one or make up your own.
| Rich | Refinery Gas | |
| Peoples | Petrol | |
| New | Naptha | |
| Kit-Kat | Kerosene | |
| Diet | Diesel | |
| Loses | Lubricating Oil | |
| Fat | Fuel Oil | |
| Rapidly | Residue |
Click here to show the Distillation of Crude Oil Experiment Video
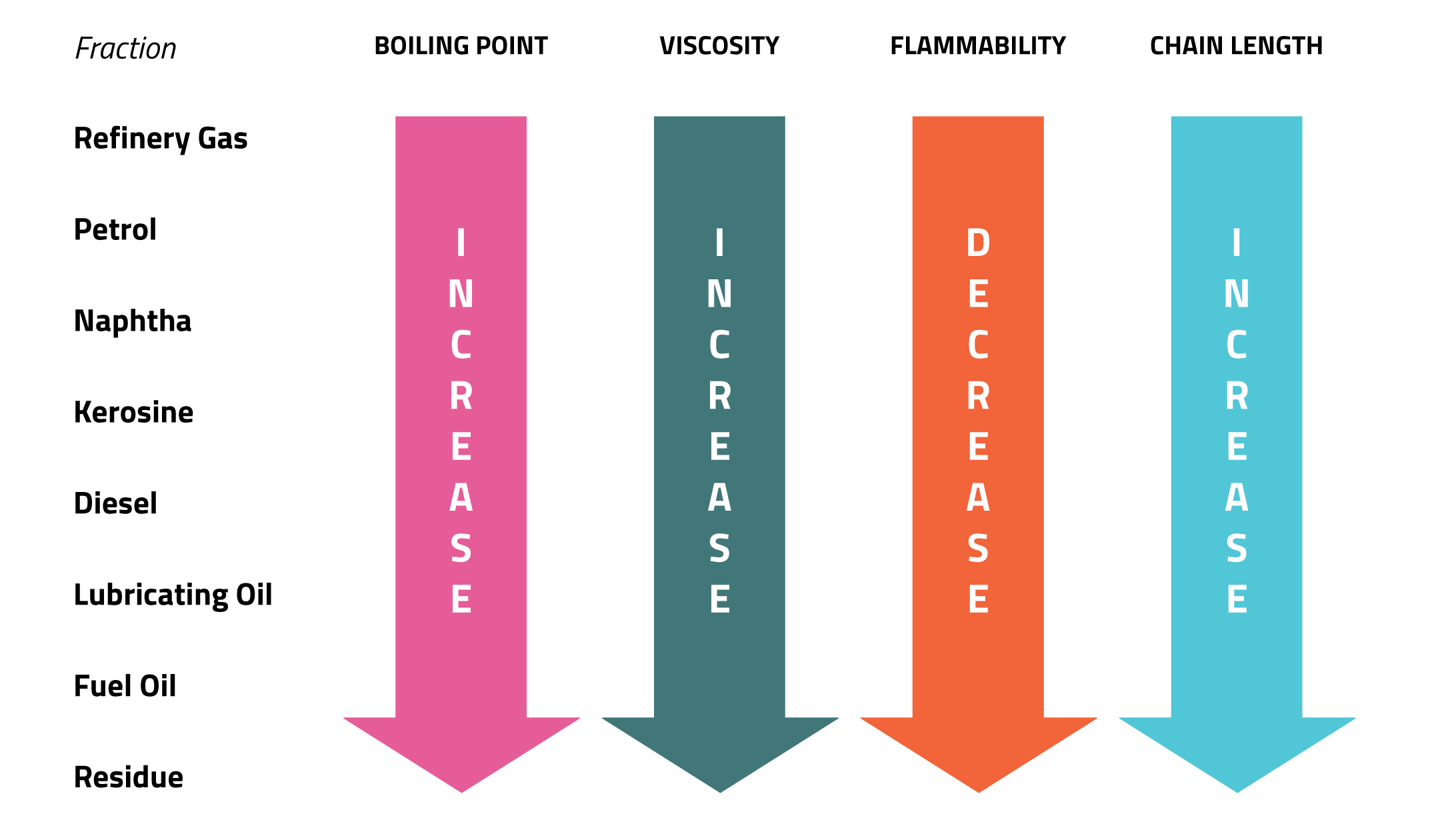
Properties of the Fractions
The fractions show a change in properties. These properties include:-
Viscosity is a measure of how thick or runny a liquid is. For example treacle has a high viscosity because it is very thick.
Flammability is a measure of how easily a substance burns.
Boiling point is the temperature that a liquid turns into a gas.
Chain length is the number of carbon atoms in each molecule. As the chain length increases the chance of incomplete combustion increases, creating more particles.
The change in these properties can be shown by the diagram below.
There is also a Homework Sheet you can download that supports and develops further the ideas from this section.
